BIOL 2404 A&P Basics
Acid Base Lab Exercise
Background:
Plant pigments called anthocyanins can be used as a color indicator to detect the pH of a solution. The color changes occur to show whether a particular solution is acidic or basic (alkaline). In nature, these plant pigments are responsible for the red, blue, and purple colors that are found in flowers, fruits, and autumn leaves.
Purpose: A solution of anthocyanins extracted from red cabbage will be used as a pH indicator to test different household products. Other natural indicators can be made from blackberries, grape juice, radish roots, and red onions.
Materials Needed:
Experiement Set up:
A. Chop half a red cabbage into small pieces
B. Put the cabbage in a saucepan and cover with water
C. Bring to a boil and boil for ~10 minutes, turn off the heat, stir the mixture and allow it cool.
D. Pour the cooled mixture through a sieve (funnel lined with gauze or collander strainer) to collect the cabbage juice into a bowl.
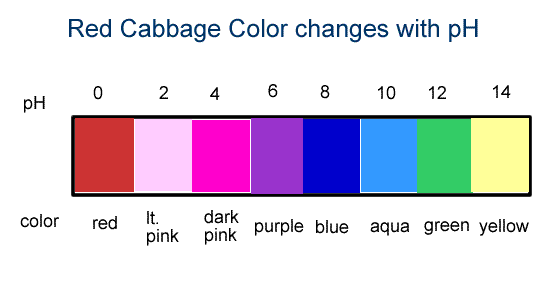
Before an indicator can be used to determine the pH of substance, the indicator's color changes at different pH values must be known. Below is the range of colors of red cabbage with varying pH.
Mix 1 tablespoon (~5 ml) of a household chemical to test and 1.5 teaspoons (~ 3ml) of red cabbage juice in a clear glass jar or cup. Match the resulting color to one on the scale below and read the pH value. Complete the following chart for the suggested household chemicals.

On the acid side of the scale, the strength of the acid increases with decreasing pH number; the lower the pH value, the stronger the acid. On the base side of the scale the strength of the base increases with increasing pH. Water is the center of the pH scale (0-14) with it's pH = 7 and is considered neutral. Remember that pH is equal to the negative log base 10 of the H+ concentration in moles per liter and mathematically expressed pH = -log [H+] m/l.
Solution |
color change seen |
pH of solution (number) |
Interpretation (acid, base, neutral) |
| distilled water | |||
| vinegar | |||
| milk | |||
| apple juice | |||
| lemon juice | |||
| carbonated soft drink | |||
| detergent water | |||
| orange juice | |||
| baking soda | |||
| milk of magnesia | |||
| coffee | |||
| bleach |
Part B:
Mix a known acidic solution with a known basic solution [do not use bleach
under any cirumstances; save the vinegar and baking soda for part C].
Retest the mixture with the red cabbage juice indicator. What is the pH? Explain
what happened.
Part C:
Mix vinegar with baking soda. Restest the pH. Explain what is happening with the bubbling seen.